Offering Choices

Our research shows that 53 percent of adults who smoke are interested in completely switching from cigarettes to a smoke-free product1 and we know that no single product will satisfy all adults who smoke.
To advance harm reduction for the millions of adults who smoke, our strategy is to deliver a compelling portfolio of satisfying, smoke-free products authorized by the Food and Drug Administration (FDA) across three of the most promising smoke-free product platforms: e-vapor, modern oral nicotine pouches, and heated tobacco products.
Product Choice and a Variety of Flavor Options are Critical to Adults Who Smoke Trying and Transitioning to Smoke-Free Products
Adults who smoke want and deserve smoke-free choices. Our research indicates that most adults who smoke believe it’s important for them to have smoke-free tobacco product choices available and that FDA should focus on making smoke-free products available to them to help them switch from cigarettes to less harmful tobacco products.2
In addition to having a selection of smoke-free products from which to choose, adult flavor varieties play an important role in transitioning adults who smoke away from combustible cigarettes. The science and evidence paint a clear picture – flavors can help adults who smoke try and switch to smoke-free products.

Five adult participants from Project 21 share the role flavors and product choice played in their transition to smoke-free products.
Published literature indicates that flavors play an important role in transitioning adults who smoke to e-vapor products. Adult e-vapor users engage with a wide variety of e-liquid flavors with strong preference for non-tobacco flavors, such as fruit, mint, or menthol flavors.3
Flavor options play an important role in continued e-vapor use after initial use, and non-tobacco flavors may play a pivotal role in discontinuation of smoking.4
Large public datasets confirm the role of flavors in transitioning and stopping smoking. For example:
- Government-sponsored researchers analyzing data from the Population Assessment of Tobacco and Health (PATH) Study found that adult smokers who used flavored e-vapor products were more than twice as likely to quit cigarette smoking than those who used non-flavored e-vapor products.
- Analysis of U.S. and Canadian data from the ITC Four Country Smoking and Vaping Survey (ITC-4CV) demonstrated that adult e-vapor users of fruit and “candy” flavors were more likely than tobacco flavor users to vape in order to quit smoking.6 Those authors concluded that “Limiting access to flavors may therefore reduce the appeal of e-cigarettes among adults who are trying to quit smoking or stay quit.” 7
- Our analysis of PATH Wave 4 dual users (cigarette and e-vapor) who transitioned to exclusive e-vapor in PATH Wave 5 found approximately 93% reported using non-tobacco flavored e-vapor products or a combination of non-tobacco and tobacco-flavored at Wave 4. The remaining approximately 7% reported using only tobacco-flavored e-vapor products at Wave 4.8
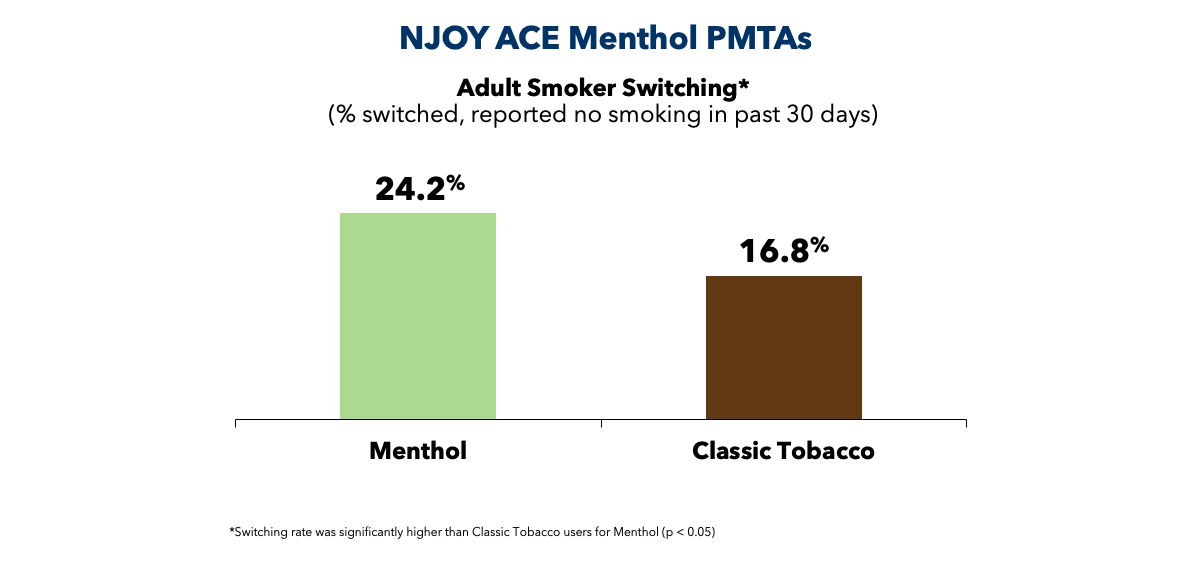
In the e-vapor category, our research demonstrates that NJOY ACE® Menthol promote substantially higher rates of complete switching after three months, relative to NJOY ACE® Classic Tobacco. The scientific evidence we submitted in our Premarket Tobacco Product Application (PMTA) also demonstrates youth use of NJOY ACE Menthol was either not detectable or exceedingly low. Based on this body of evidence, in June 2024, the FDA issued marketing granted orders (MGOs) for NJOY ACE Pod Menthol 2.4% and 5%, NJOY DAILY Menthol 4.5%, and NJOY DAILY Extra Menthol 6%.
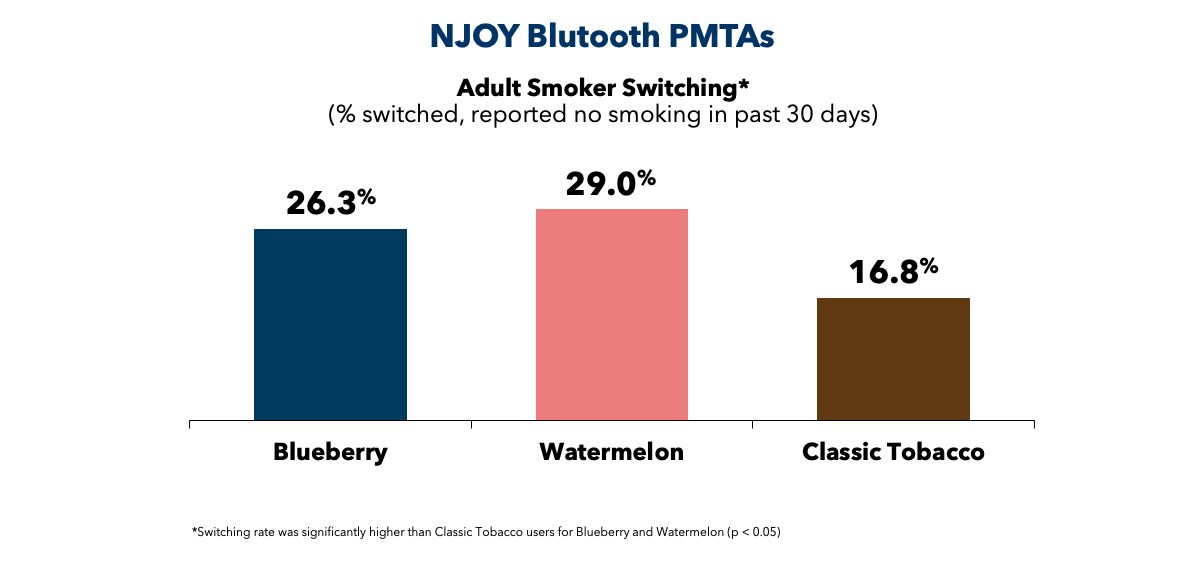
In May 2024, NJOY submitted a supplemental PMTA for the NJOY ACE 2.0 device featuring Bluetooth®-enabled access restriction technology. The company also re-submitted PMTAs for Blueberry and Watermelon pod products that work exclusively with the NJOY ACE 2.0 device. Similar to NJOY ACE Menthol, our data show NJOY ACE 2.0 flavored products are more effective at promoting adult switching relative to NJOY ACE Classic Tobacco. In addition, we’ve demonstrated the age-gating restrictions are effective at preventing underage access in virtually all cases.
In the modern oral nicotine pouch category, in support of our PMTA applications for on!® , we conducted an actual use study (AUS) that characterized product use, its influence on other tobacco use, and the potential role of flavor variety on behaviors.9 The study involved 6-weeks ad libitum use of multiple varieties of on!. 1,147 adult tobacco users not planning to quit cigarettes and/or smokeless tobacco completed the study.
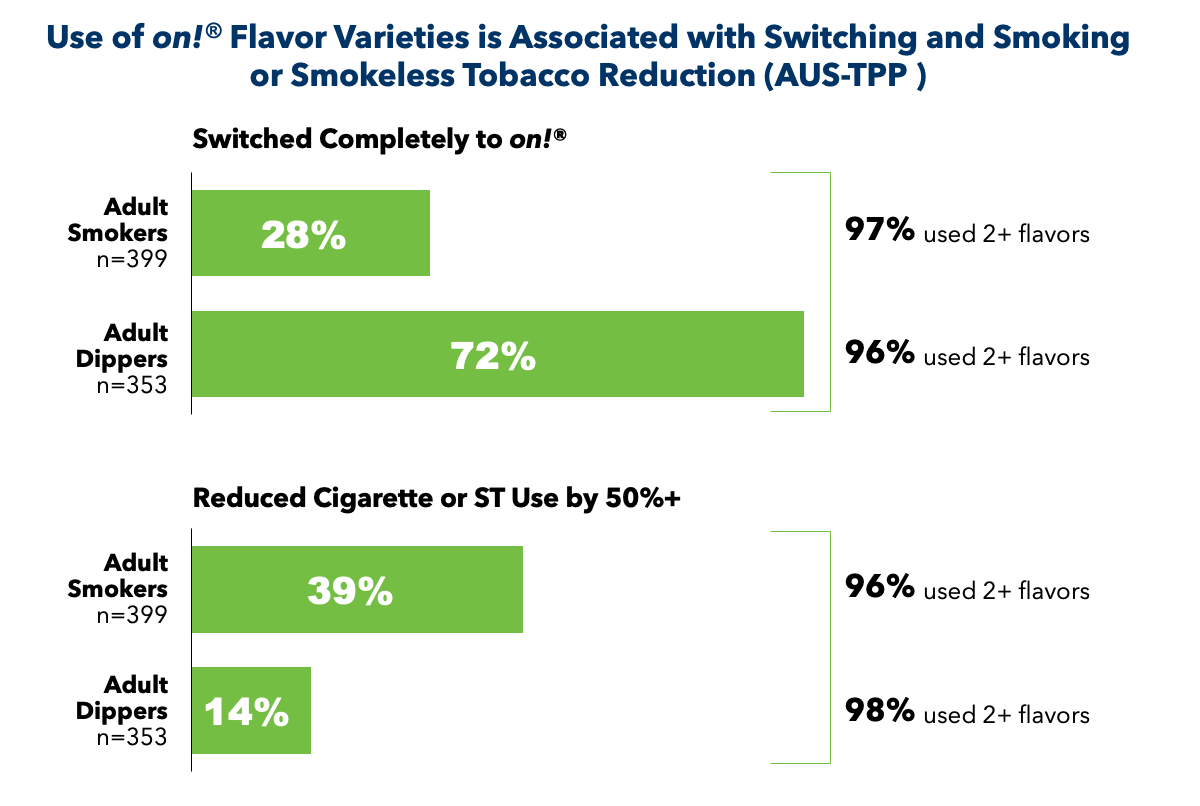
We found that 90% of participants used more than one flavor throughout the course of the study. Over 80% of adult tobacco consumers who switched from cigarettes to on! pouches used three or more flavors. Moreover, an increase in the number of flavors used for on! pouches was associated with a decrease in cigarette consumption. For adults who smoke, the observed complete switching rate was approximately 28% and nearly 72% of primary adults who dip switched to on! pouches from smokeless tobacco.10 Further analysis of the switchers, and those who reduced smoking or smokeless tobacco use by 50% or more in this study, showed that 96-98% used 2 or more flavors.
The journey for adults who smoke to smoke-free products is complicated, personal and unique. And along their journey, there will be motivators as well as barriers to switch to smoke-free products. One key motivator that helps adults who smoke start and continue their journey is having access to a portfolio of satisfying, smoke-free products authorized by FDA. By providing adults who smoke what they deserve and want, we can advance harm reduction for the millions of adults who smoke.